What Methods Are Used to Measure Ph Briefly Describe
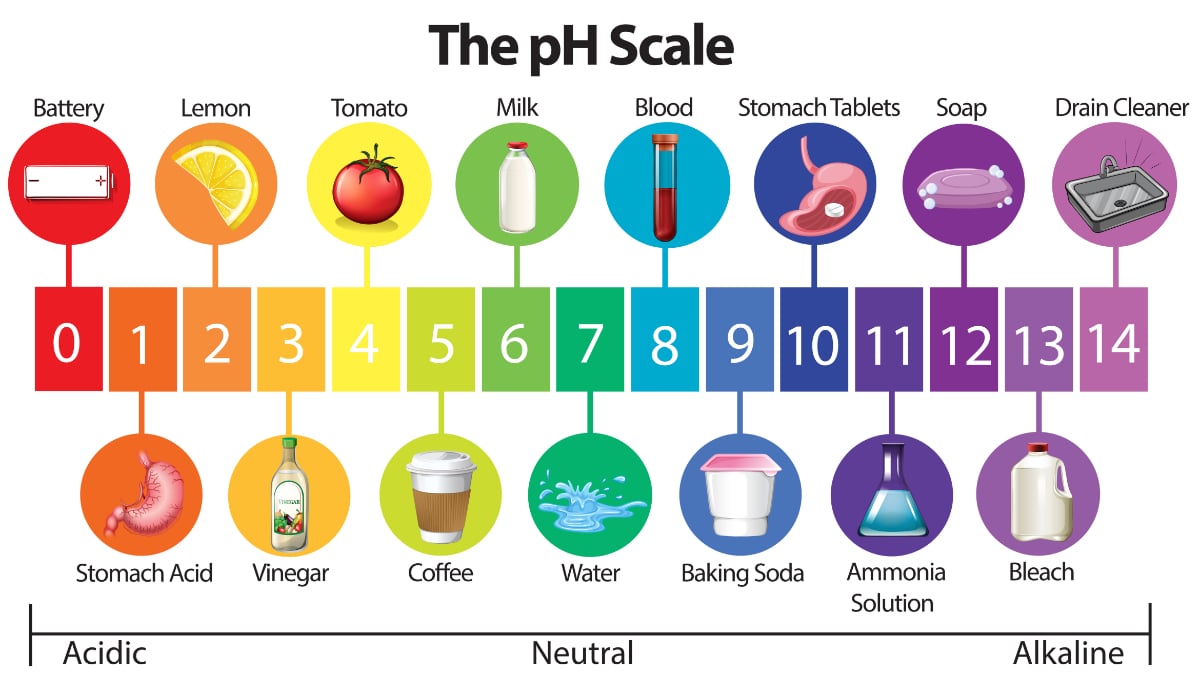
Conventionally the pH is checked by pH paper or litmus paper. PH of acids is generally less than 7 whereas for bases it is greater than 7.
The color is compared to a reference card that shows the pH.
. The potential of the combination cell in millivolts is directly related to the pH of the solution. What are three methods used to measure pH. Electrode potential E E slope pH 1.
Together with a troubleshooting diagram they give the information needed in order to ensure the correct working of the pH electrodes for long periods of time. The most precise of the 3 test options pH meters measure a solutions pH by measuring the electrical potential difference between the pH electrode and a reference electrode. Thus a soil of pH 50 has 10 times more H ion in soil solution than a.
This category basically includes two methods. The meter then coverts this potential to a pH reading. Lemon Juice Hand Sanitizer SoapDishwashing Liquid Predicted pH Cabbage Indicator Color pH range low - high Acidic Basic or Neutral Analysis.
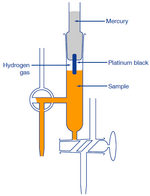
A hydrogen electrode is made by adding platinum black to platinum wire or a platinum. ANSWER EACH QUESTIONS BRIEFLY. K w H 3 O OH 10 14.
They change colors at certain pH ranges and they dont give a certain pH number How do acid-base indicators work. It measures the voltage electrical potential produced by the solution whose acidity were interested in compares it with the voltage of a known solution and uses the difference in voltage the potential difference between them to deduce the difference in pH. You will generally need more than one buffer for calibrating a pH meter.
Generally by just looking at the structure of drugs how can you determine if the drug is basic or acidic. For now pH measurements will be made using an electrochemical cell with both the sensing pH and reference electrode built into a single combination electrode. A pH-meter is an instrument used for the determination of pH.
Indicator methods Metal-electrode methods including the hydrogen-electrode method quinhydron-electrode method and antimony-electrode method Glass-electrode methods Semiconductor sensor methods Then each measuring method is explained briefly. 1 Measuring pH using an indicator This category basically includes two methods. Thus the pH of an acidic solution of HNO 3 10 3 M 3 a basic solution of KOH having OH 10 4 M and H 3 O 10 10 M will have a pH 10.
What does the term drug ionized and. Ah we can use pH paper which changes a ah to a particular color based on the pH. This category basically includes two methods.
Ways of Measuring pH 1 Measuring pH Using an Indicator. The change in litmus paper color indicates the change in pH it is called as visual method. Solutions with a pH greater than 7 are alkaline If universal indicator is added to a solution it changes to a colour that shows the pH of the solution.
Briefly describe how different methods can be used to indicate pH of a sample. The first will be a neutral buffer with a pH of 7 and the second should be near the expected sample pH either a pH of 4 or 921. How does pH affect drug solubility.
At 298 K ionic product of water K w can be given as. Or you can use an electrode to measure the pH and the ah electrodes sends a current and based on the r. A hydrogen electrode is made by adding platinum black to platinum wire or a platinum.
What are the different methods of measuring pH. The methods for measuring pH fall roughly into the following four categories. Refer to the experiment and gathered data to.
Inside the glass is an internal standard acidity solution usually 01 M HCl along with an internal reference electrode RE in typically an AgAgCl wire electrode. This paper has been infused with several acid-base indicators and turn into different colors depending on the pH of the solution. Buffers with a higher pH 921 are best for measuring bases whereas buffers with a low pH 4 are best for measuring acidic samples.
What are three safe. The booklets describe the pH meters and electrodes and the other elements that constitute a pH measurement system. Using a pH Meter The standard methodology for measuring pH pH meters consist of a glass electrode made of a specialty glass membrane that is sealed at the end forming a bulb.
What is the importance of pH determination in pharmacy. One involves comparing the standard. One involves comparing the standard color corresponding to a known pH with the color of an indicator immersed in the test liquid using buffer solution.
One involves comparing the standard. Whats it made of. A pH meter takes advantage of this and works like a voltmeter.
The H ion concentration has a ten fold change between each whole pH number. PH is a measure of the acidityalkalinity of a solution. Taking the negative logarithm of RHS and LHS we deduce.
For precise noting of pH an instrument is used and it is called as pH meter. Ways of Measuring pH 1 Measuring pH Using an Indicator.

Water Quality 101 What Is Ph In Water Testing

Determining Ph Methods Classification Video Lesson Transcript Study Com


No comments for "What Methods Are Used to Measure Ph Briefly Describe"
Post a Comment